Description
Kedun SteriPS Filter Cartridges are specially designed to provide superior flow rates at an economical cost. The unique construction allows for optimal sterilization effect with low diffusion flow. Hydrophilic polyethersulfone membrane cartridges require no pre-wetting and are ready to use.This assures a cost effective device while maintaining excellent performance in the pharmaceutical application.
Features and Benefits
- Low diffusion flow
- Inherently hydrophilic PES membrane
- High surface area design provides excellent flow rates and extended service life while maintaining high bacteria removal efficiency
- Lower protein binding
Validation Guide
The comprehensive validation guide is available upon request.
Materials of Construction
| Filter Medium | Polyethersulfone |
| Support | Polypropylene |
| Core/Cage/End Caps | Polypropylene |
| Effective Filter Area | SPSH 0.66 m2 / Ф69-10 inch |
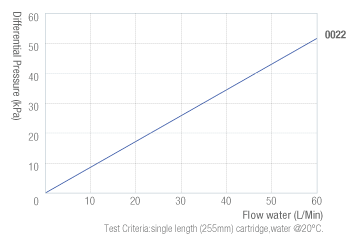
Operating Conditions
| Maximum Temperature | 90°C |
| Max.Differential Pressure |
4 bar / 21°C 2.4 bar / 70°C |
|
Steam Sterilization (Autoclave) |
121°C / 30min |
Integrity Testing Parameters
| Bubble Point |
|
≥3.2bar in water @23°C |
| Diffusion Flow | Cobetter SPSH | ≤25ml/min @2750mbar |
| P Company | Supor EBV ≤23ml/min @2760mbar | |
| Supor UEAV ≤50ml/min @2760mbar | ||
| M Company | ≤30ml/min 2750mbar |
Quality and Biological Safety
- 100% integrity tested
- Manufactured under a quality management system certified toISO9001:2008
- Meets USP Biological Reactivity Test, in vivo, for Class VI-121 °C Plastics
- Every filter tested during manufacture. Test correlated to microbial retention
- Bacterial Endotoxins (< 0.25 EU/mL)
- Extractables< 30mg per 10 inch
-
Certificate of Test provided includes:
- Fabrication Integrity
- Bacterial Retention
- Materials of constructions
- Effluent quality for cleanliness, TOC and Water Conductivity, pH and Pyrogens
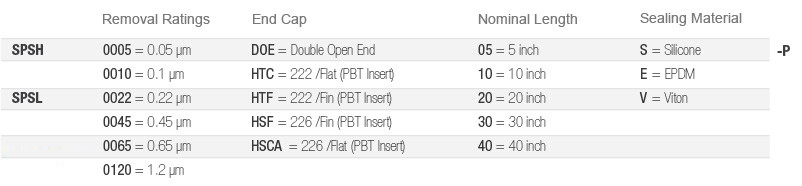
Ordering Information